Scott Rata
DPhil Student

I am a fourth-year DPhil student, modelling the G2/M transition with Helfrid Hochegger’s group. We are investigating the factors that contribute to the triggering and to the hysteresis of this transtion.
My research interests also include the metaphase-to-anaphase transition, modelling the regulation of separase and its effects on the localisation of the chromosomal passenger complex.
Publications contributed to in the context of this project
-
Thursday, Nov 15, 2018
Current Biology
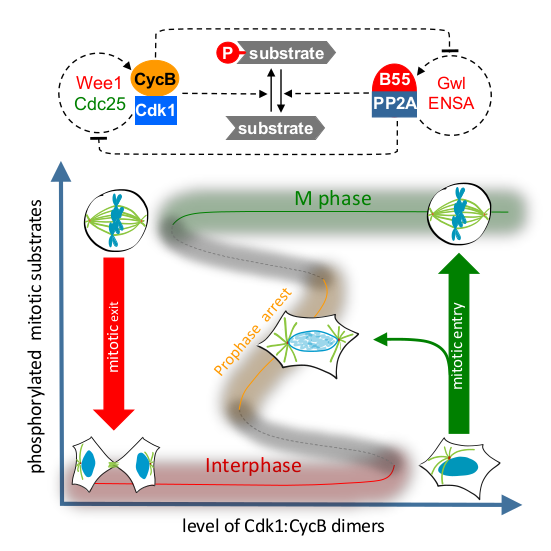
Two Interlinked Bistable Switches Govern Mitotic Control in Mammalian Cells
Scott Rata, Maria F. Suarez Peredo Rodriguez, Stephy Joseph, Nisha Peter, Fabio E. Iturra, Fengwei Yang, Anotida Madzvamuse, Jan G. Ruppert, Kumiko Samejima, Melpomeni Platani, Monica Alvarez-Fernandez, Marcos Malumbres, William C. Earnshaw, Bela Novak, Helfrid Hochegger
-
AbstractDistinct protein phosphorylation levels in interphase and M phase require tight regulation of Cdk1 activity [1,2]. A bistable switch, based on positive feedback in the Cdk1 activation loop, has been proposed to generate different thresholds for transitions between these cell-cycle states [3–5]. Recently, the activity of the major Cdk1-counteracting phosphatase, PP2A:B55, has also been found to be bistable due to Greatwall kinase-dependent regulation [6]. However, the interplay of the regulation of Cdk1 and PP2A:B55 in vivo remains unexplored. Here, we combine quantitative cell biology assays with mathematical modeling to explore the interplay of mitotic kinase activation a nd phosphatase inactivation in human cells. By measuring mitotic entry and exit thresholds using ATP-analog-sensitive Cdk1 mutants, we find evidence that the mitotic switch displays hysteresis and bistability, responding differentially to Cdk1 inhibition in the mitotic and interphase states. Cdk1 activation by Wee1/Cdc25 feedback loops and PP2A:B55 inactivation by Greatwall independently contributes to this hysteretic switch system. However, elimination of both Cdk1 and PP2A:B55 inactivation fully abrogates bistability, suggesting that hysteresis is an emergent property of mutual inhibition between the Cdk1 and PP2A:B55 feedback loops. Our model of the two interlinked feedback systems predicts an intermediate but hidden steady state between interphase and M phase. This could be verified experimentally by Cdk1 inhibition during mitotic entry, supporting the predictive value of our model. Furthermore, we demonstrate that dual inhibition of Wee1 and Gwl kinases causes loss of cell-cycle memory and synthetic lethality, which could be further exploited therapeutically.
-
Tuesday, Sep 19, 2017
Cell Cycle
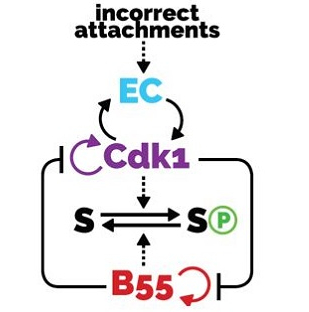
Interlinked bistable mechanisms generate robust mitotic transitions
Lukas Hutter, Scott Rata, Helfrid Hochegger, Bela Novak
-
AbstractThe transitions between phases of the cell cycle have evolved to be robust and switch-like, which ensures temporal separation of DNA replication, sister chromatid separation, and cell division. Mathematical models describing the biochemical interaction networks of cell cycle regulators attribute these properties to underlying bistable switches, which inherently generate robust, switch-like, and irreversible transitions between states. We have recently presented new mathematical models for two control systems that regulate crucial transitions in the cell cycle: mitotic entry and exit and the mitotic checkpoint.Each of the two control systems is characterized by two interlinked bistable switches. In the case of mitotic checkpoint control, these switches are mutually activating, whereas in the case of the mitotic entry/exit network, the switches are mutually inhibiting. In this Perspective we describe the qualitative features of these regulatory motifs and show that having two interlinked bistable mechanisms further enhances robustness and irreversibility. We speculate that these network motifs also underlie other cell cycle transitions and cellular transitions between distinct biochemical states.
-
Wednesday, Nov 23, 2016
Current Biology
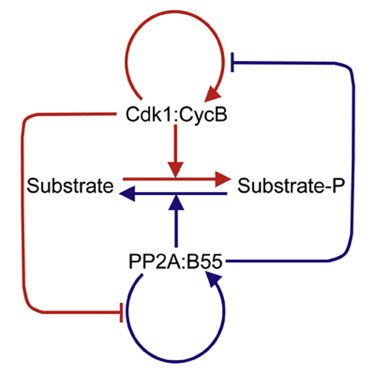
Two Bistable Switches Govern M Phase Entry
Satoru Mochida, Scott Rata, Hirotsugu Hino, Takeharu Nagai, Bela Novak
-
AbstractThe abrupt and irreversible transition from interphase to M phase is essential to separate DNA replication from chromosome segregation. This transition requires the switch-like phosphorylation of hundreds of proteins by the cyclin-dependent kinase 1 (Cdk1):cyclin B (CycB) complex. Previous studies have ascribed these switch-like phosphorylations to the auto-activation of Cdk1:CycB through the removal of inhibitory phosphorylations on Cdk1-Tyr15 [1 and 2]. The positive feedback in Cdk1 activation creates a bistable switch that makes mitotic commitment irreversible [2, 3 and 4]. Here, we surprisingly find that Cdk1 auto-activation is dispensable for irreversible, switch-like mitotic entry due to a second mechanism, whereby Cdk1:CycB inhibits its counteracting phosphatase (PP2A:B55). We show that the PP2A:B55-inhibiting Greatwall (Gwl)-endosulfine (ENSA) pathway is both necessary and sufficient for switch-like phosphorylations of mitotic substrates. Using purified components of the Gwl-ENSA pathway in a reconstituted system, we found a sharp Cdk1 threshold for phosphorylation of a luminescent mitotic substrate. The Cdk1 threshold to induce mitotic phosphorylation is distinctly higher than the Cdk1 threshold required to maintain these phosphorylations—evidence for bistability. A combination of mathematical modeling and biochemical reconstitution show that the bistable behavior of the Gwl-ENSA pathway emerges from its mutual antagonism with PP2A:B55. Our results demonstrate that two interlinked bistable mechanisms provide a robust solution for irreversible and switch-like mitotic entry.
-
Friday, May 27, 2016
BioEssays
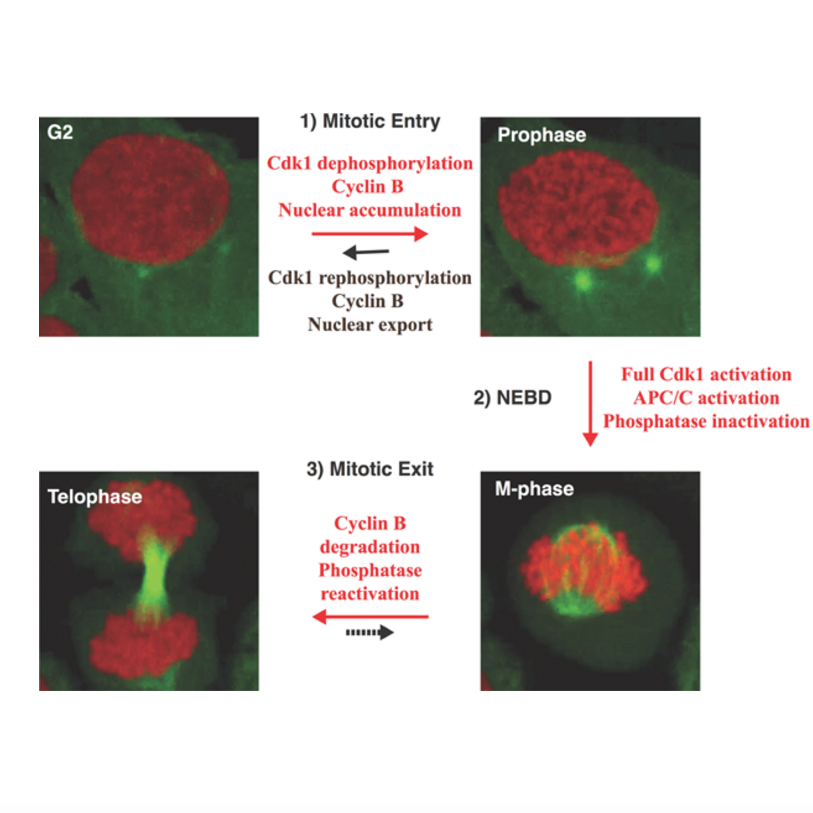
Bistability of mitotic entry and exit switches during open mitosis in mammalian cells
Nadia Hegarat, Scott Rata, Helfrid Hochegger
-
AbstractMitotic entry and exit are switch-like transitions that are driven by the activation and inactivation of Cdk1 and mitotic cyclins. This simple on/off reaction turns out to be a complex interplay of various reversible reactions, feedback loops, and thresholds that involve both the direct regulators of Cdk1 and its counteracting phosphatases. In this review, we summarize the interplay of the major components of the system and discuss how they work together to generate robustness, bistability, and irreversibility. We propose that it may be beneficial to regard the entry and exit reactions as two separate reversible switches that are distinguished by differences in the state of phosphatase activity, mitotic proteolysis, and a dramatic rearrangement of cellular components after nuclear envelope breakdown, and discuss how the major Cdk1 activity thresholds could be determined for these transitions.