The “bicycle” project
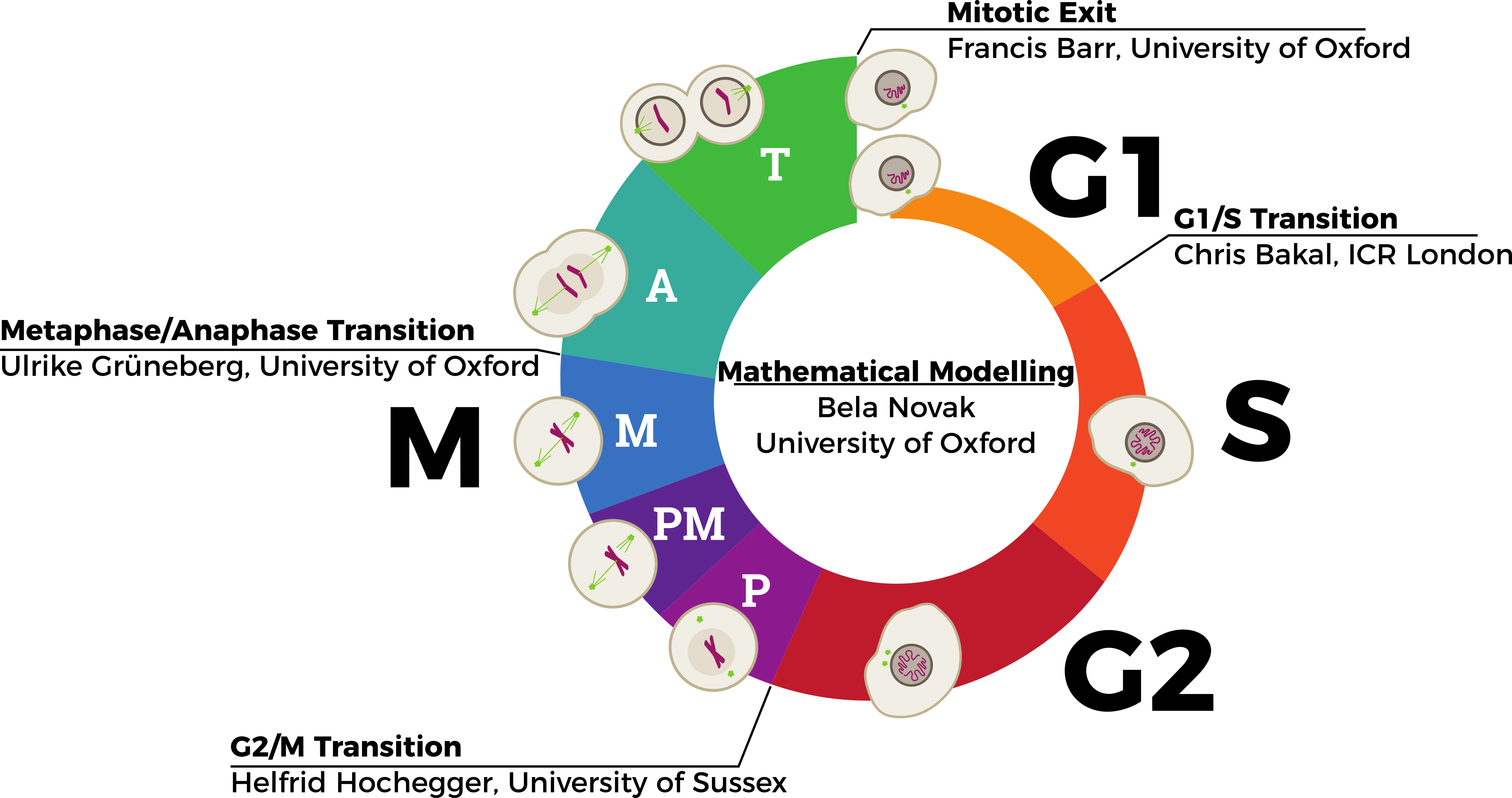
During human development and growth, cells must proliferate in an ordered and controlled manner to form the adult body. The cell cycle is a series of events that results in two cells forming from one parent cell. Importantly each daughter cell must be identical to the parent cell, thus successful completion of the cell cycle requires the precise copying and distribution of genetic information contained within DNA. Cell cycle events are orchestrated by a large array of proteins that interact and function together to ensure the cell cycle is precisely controlled. Molecular biologists have uncovered many of these cell cycle proteins. Remarkably, these proteins and how they interact are highly conserved from yeasts to humans. However because the system is very complex, it is not obvious how all these proteins work together to drive the cell cycle forward. In order to use this hard-won information about the mechanisms controlling the cell cycle, computational tools are necessary to reliably simulate the integrated behaviour of the system. Mathematical models of relatively simple, cell-cycle control systems in yeasts and early embryos revealed that the cell cycle control network has the property of a “switch-like” system. These effects make the transitions between cell cycle states abrupt (switch-like), unidirectional (irreversible) and controllable (sensitive to error-detection mechanisms). When the cell detects a problem with the execution of a particular process, it can block the transition to the next cell cycle stage by inhibiting the switch. This switch-like behaviour has been demonstrated experimentally for all cell cycle transitions in single-celled organisms (e.g. yeasts). However it is still unknown whether these principles are also manifested in the cell cycle control of more complex cells (e.g. in humans). Therefore in this hypothesis-driven research programme, we propose to test the design principles of more complex cell cycle control network of human cells.

We will use a combination of high-tech experimental and theoretical tools to tackle this problem. In order to achieve this goal, an interdisciplinary team of biochemists, cell biologists and modellers will be assembled. Understanding the principles of cell cycle controls in mammalian cells is vital to the understanding of human disease. For example, in cancer, cells lose control of the cell cycle and replicate repeatedly, forming a tumour. By understanding how the cell cycle is controlled, we can develop new ways to restore cell cycle control to cancer cells and stop them replicating out of control. In contrast, if the cellular reproduction of stem cells in the body is compromised, renewal of differentiated cells is retarded which is one of the reasons underlying the ageing process. Since many emerging stem cell therapies rely on the precise control of cell replication this field will also benefit from our research.





