Ulrike Gruneberg
Principal Investigator

Ulrike Gruneberg is a Senior Medical Research Fellow at the Sir William Dunn School of Pathology in Oxford. Ulrike received her postdoctoral training in the labs of Elmar Schiebel and Erich Nigg and was subsequently awarded a prestigious Cancer Research UK Career Development Fellowship to start her own lab, initially at the University of Liverpool and then at the University of Oxford. Ulrike’s lab studies the mechanisms that regulate spindle formation and chromosome segregation in mammalian cells. In particular, Ulrike is interested in understanding the process of the metaphase-to-anaphase transition and the regulatory mechanisms that control this cell cycle transition. Questions that are studied in the lab are how stable microtubule-kinetochore interactions are formed, how the spindle assembly checkpoint is activated and silenced and how dynamic post-translational modifications such as phosphorylation and acetylation control these cell cycle events. Ulrike uses a combination of cell biological and biochemical techniques to address these questions, and the lab has extensive expertise in the generation and use of fluorescently tagged stable cell lines to study cell cycle progression by live cell imaging, as well as the implementation of biochemical and immunofluorescence based screens to identify novel regulators of cell cycle progression.
Publications contributed to in the context of this project
-
Wednesday, Jan 23, 2019
Journal of Cell Biology
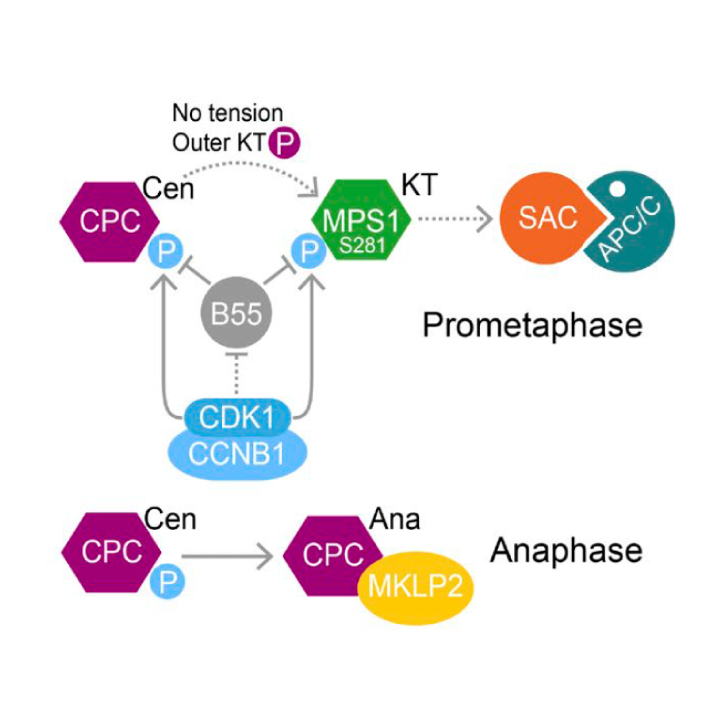
CDK1-CCNB1 creates a spindle checkpoint–permissive state by enabling MPS1 kinetochore localization
Daniel Hayward, Tatiana Alfonso-Pérez, Michael J. Cundell, Michael Hopkins, James Holder, James Bancroft, Lukas H. Hutter, Bela Novak, Francis A. Barr, Ulrike Gruneberg
-
AbstractSpindle checkpoint signaling is initiated by recruitment of the kinase MPS1 to unattached kinetochores during mitosis. We show that CDK1-CCNB1 and a counteracting phosphatase PP2A-B55 regulate the engagement of human MPS1 with unattached kinetochores by controlling the phosphorylation status of S281 in the kinetochore-binding domain. This regulation is essential for checkpoint signaling, since MPS1S281A is not recruited to unattached kinetochores and fails to support the recruitment of other checkpoint proteins. Directly tethering MPS1S281A to the kinetochore protein Mis12 bypasses this regulation and hence the requirement for S281 phosphorylation in checkpoint signaling. At the metaphase–anaphase transition, MPS1 S281 dephosphorylation is delayed because PP2A-B55 is negatively regulated by CDK1-CCNB1 and only becomes fully active once CCNB1 concentration falls below a characteristic threshold. This mechanism prolongs the checkpoint-responsive period when MPS1 can localize to kinetochores and enables a response to late-stage spindle defects. By acting together, CDK1-CCNB1 and PP2A-B55 thus create a spindle checkpoint–permissive state and ensure the fidelity of mitosis.
-
Wednesday, Jan 23, 2019
Journal of Cell Biology
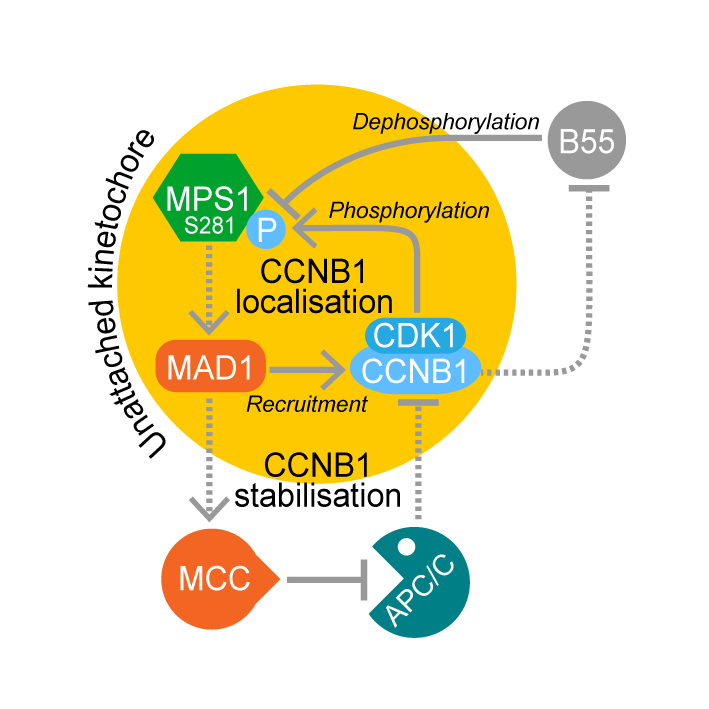
MAD1-dependent recruitment of CDK1-CCNB1 to kinetochores promotes spindle checkpoint signaling
Tatiana Alfonso-Pérez, Daniel Hayward, James Holder, Ulrike Gruneberg, Francis A. Barr
-
AbstractCyclin B–dependent kinase (CDK1-CCNB1) promotes entry into mitosis. Additionally, it inhibits mitotic exit by activating the spindle checkpoint. This latter role is mediated through phosphorylation of the checkpoint kinase MPS1 and other spindle checkpoint proteins. We find that CDK1-CCNB1 localizes to unattached kinetochores and like MPS1 is lost from these structures upon microtubule attachment. This suggests that CDK1-CCNB1 is an integral component and not only an upstream regulator of the spindle checkpoint pathway. Complementary proteomic and cell biological analysis demonstrate that the spindle checkpoint protein MAD1 is one of the major components of CCNB1 complexes, and that CCNB1 is recruited to unattached kinetochores in an MPS1-dependent fashion through interaction with the first 100 amino acids of MAD1. This MPS1 and MAD1-dependent pool of CDK1-CCNB1 creates a positive feedback loop necessary for timely recruitment of MPS1 to kinetochores during mitotic entry and for sustained spindle checkpoint arrest. CDK1-CCNB1 is therefore an integral component of the spindle checkpoint, ensuring the fidelity of mitosis.