Nadia Hegarat
Postdoc

Nadia is interested in the link between cell cycle and genome stability. In cycling cells, the maintenance of genome integrity consists in faithful genome duplication, accurate segregation of the replicated chromosomes and repair of damaged DNA. She started her research career in France (“Régulation et dynamique des génomes”) where she developed a highly specific purification method of DNA repair complexes. In 2008, she joined Helfrid Hochegger’s group in the University of Sussex as a post-doctoral fellow to expand on her interest in cell cycle regulation. She worked on Aurora kinase A, an essential mitotic kinase with unclear functions. She found that Aurora A regulates chromosome segregation by controlling microtubule depolymerisation during the spindle disassembly. They also made an advance in the understanding of how anaphase onset is initiated: they identified protein phosphatases controlling the mitotic exit regulators Greatwall and ENSA. Her current research project is to understand how cyclins contributes to G2/M transition and mitotic progression and she is working closely with Bela Novak’s team to tackle this question.
Publications contributed to in the context of this project
-
Thursday, Apr 30, 2020
EMBO Journal
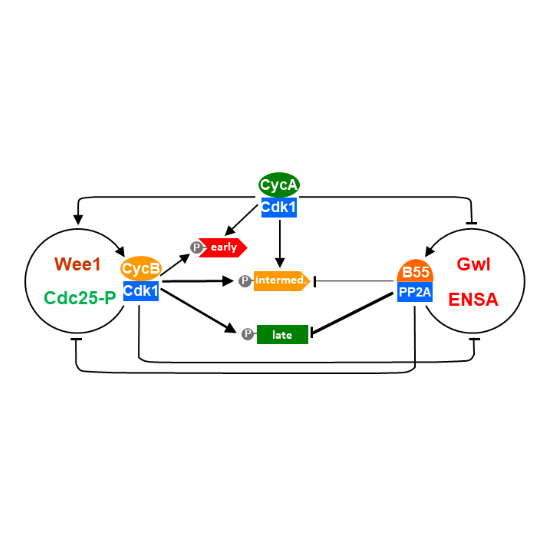
Cyclin A Triggers Mitosis Either via the Greatwall Kinase Pathway or Cyclin B
Nadia Hegarat, Adrijana Crncec, Maria F Suarez Peredo Rodriguez, Fabio Echegaray Iturra, Yan Gu, Oliver Busby, Paul F Lang, Alexis R Barr, Chris Bakal, Masato T Kanemaki, Angus I Lamond, Bela Novak, Tony Ly, Helfrid Hochegger
-
AbstractTwo mitotic cyclin types, cyclin A and B, exist in higher eukaryotes, but their specialised functions in mitosis are incompletely understood. Using degron tags for rapid inducible protein removal, we analyse how acute depletion of these proteins affects mitosis. Loss of cyclin A in G2-phase prevents mitotic entry. Cells lacking cyclin B can enter mitosis and phosphorylate most mitotic proteins, because of parallel PP2A:B55 phosphatase inactivation by Greatwall kinase. The final barrier to mitotic establishment corresponds to nuclear envelope breakdown, which requires a decisive shift in the balance of cyclin-dependent kinase Cdk1 and PP2A:B55 activity. Beyond this point, cyclin B/Cdk1 is essential for phosphorylation of a distinct subset of mitotic Cdk1 substrates that are essential to complete cell division. Our results identify how cyclin A, cyclin B and Greatwall kinase coordinate mitotic progression by increasing levels of Cdk1-dependent substrate phosphorylation.
-
Wednesday, Aug 1, 2018
Molecular Cell
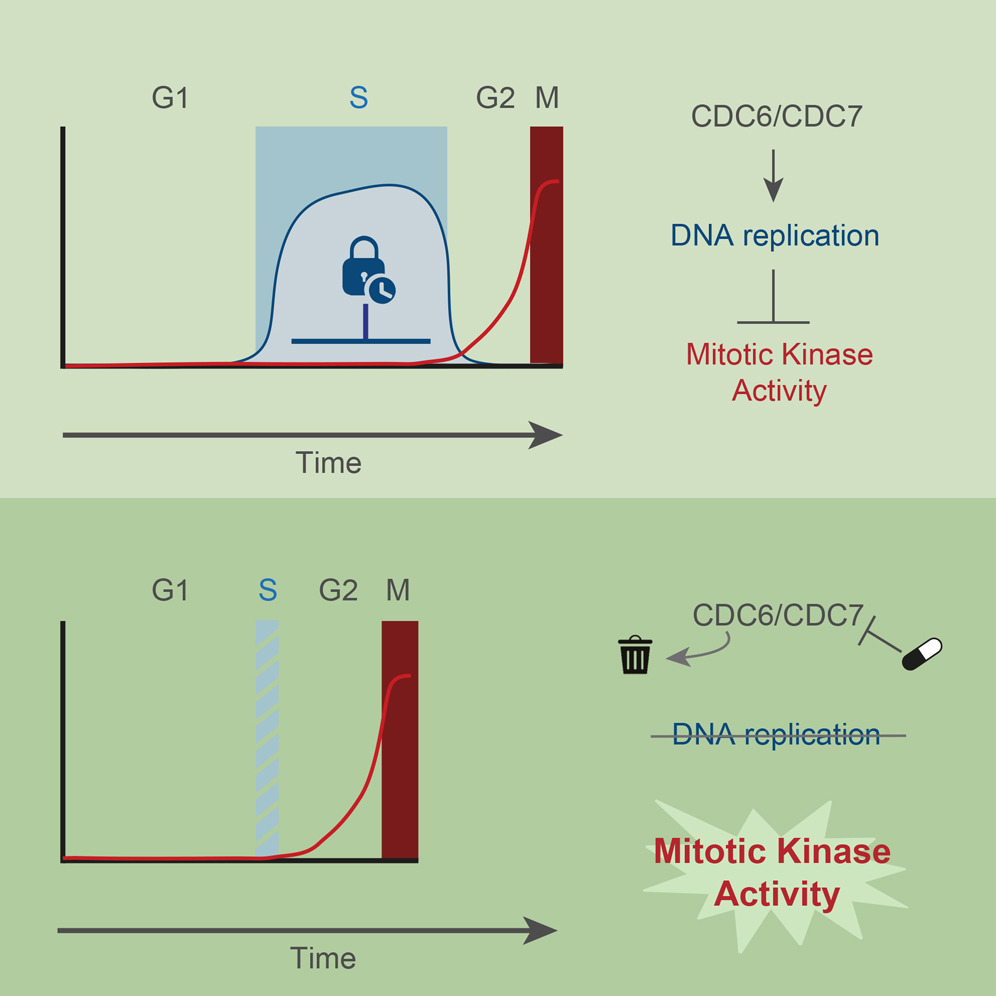
DNA Replication Determines Timing of Mitosis by Restricting CDK1 and PLK1 Activation
Bennie Lemmens, Nadia Hegarat, Karen Akopyan, Joan Sala-Gaston, Jiri Bartek, Helfrid Hochegger, Arne Lindqvist
-
AbstractTo maintain genome stability, cells need to replicate their DNA before dividing. Upon completion of bulk DNA synthesis, the mitotic kinases CDK1 and PLK1 become active and drive entry into mitosis. Here, we have tested the hypothesis that DNA replication determines the timing of mitotic kinase activation. Using an optimized double-degron system, together with kinase inhibitors to enforce tight inhibition of key proteins, we find that human cells unable to initiate DNA replication prematurely enter mitosis. Preventing DNA replication licensing and/or firing causes prompt activation of CDK1 and PLK1 in S phase. In the presence of DNA replication, inhibition of CHK1 and p38 leads to premature activation of mitotic kinases, which induces severe replication stress. Our results demonstrate that, rather than merely a cell cycle output, DNA replication is an integral signaling component that restricts activation of mitotic kinases. DNA replication thus functions as a brake that determines cell cycle duration.
-
Friday, May 27, 2016
BioEssays
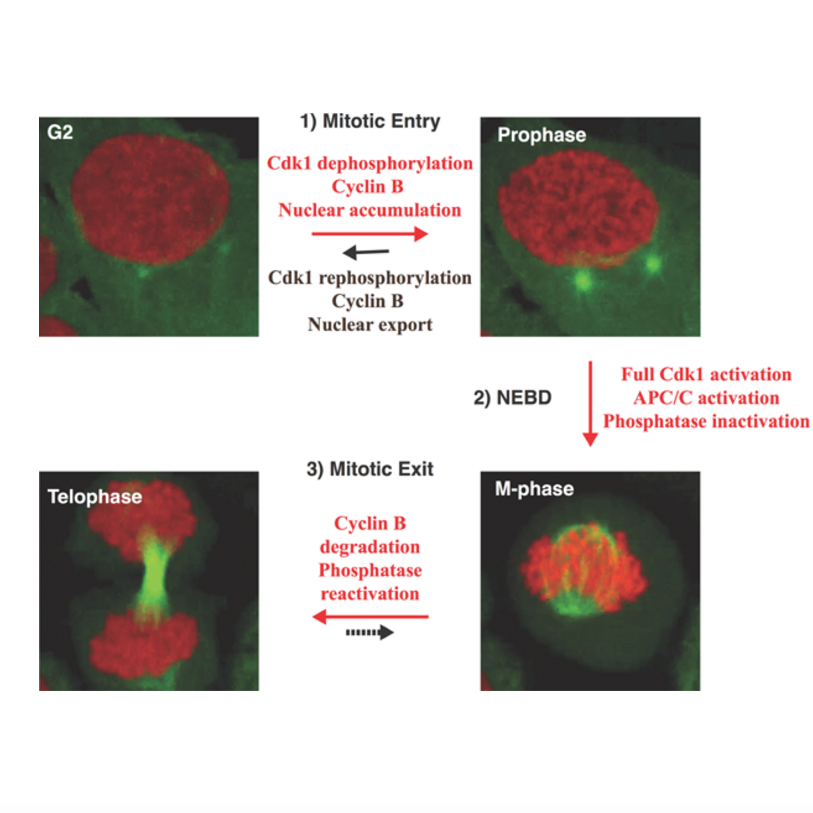
Bistability of mitotic entry and exit switches during open mitosis in mammalian cells
Nadia Hegarat, Scott Rata, Helfrid Hochegger
-
AbstractMitotic entry and exit are switch-like transitions that are driven by the activation and inactivation of Cdk1 and mitotic cyclins. This simple on/off reaction turns out to be a complex interplay of various reversible reactions, feedback loops, and thresholds that involve both the direct regulators of Cdk1 and its counteracting phosphatases. In this review, we summarize the interplay of the major components of the system and discuss how they work together to generate robustness, bistability, and irreversibility. We propose that it may be beneficial to regard the entry and exit reactions as two separate reversible switches that are distinguished by differences in the state of phosphatase activity, mitotic proteolysis, and a dramatic rearrangement of cellular components after nuclear envelope breakdown, and discuss how the major Cdk1 activity thresholds could be determined for these transitions.